Your Global Partner in Pharmaceutical Contamination Control
Sterility Assurance is not an isolated exercise. At Particle Measuring Systems we understand that finding the right partner is key to a less stressful solution, especially for pharmaceutical contamination control. Critical instrumentation used for measurement must be accurate, but more than just accurate, the data must support the whole process. Using the data, allows our team of experts within the PMS® Advisory Services to align your processes with the regulatory demands of EU GMP Annex 1, ISO 14644-1 / 2, USP 788, USP 797, 21 CFR Part 11 and ISO 21501-4 and more.
Data trending, the assignment of appropriate levels and limits and then how best to react to an out-of-tolerance event can all be supported by helping you understand the data to move beyond compliance to ensure environmental control and continuous improvement, accelerating change and innovation.
Plus, our teams are direct in every major geography, providing you with peace of mind no matter where your offices are located.
Complete Sterility Assurance Solutions
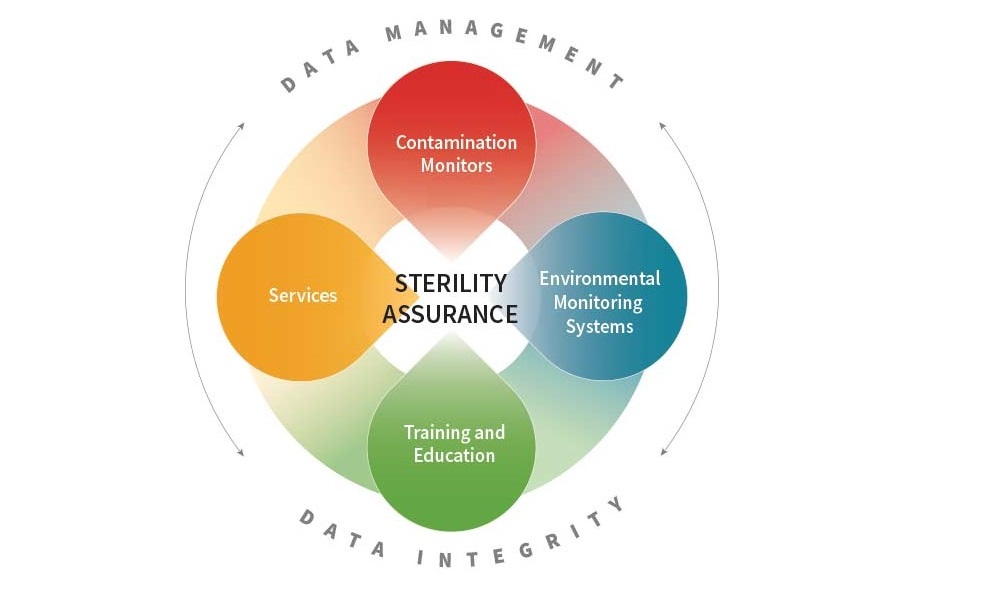
Our complete particle monitoring solutions provide you with the instruments, software, knowledge, and quality service for your seamless application needs.
We provide the complete solutions you need including:
- Contamination Monitors – Particle Counters & Microbial instruments for remote & portable applications
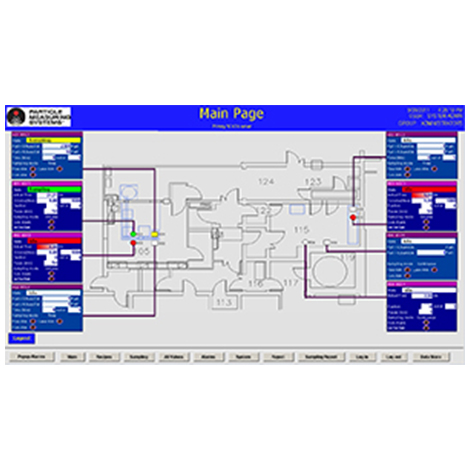
- Environmental Monitoring Systems – integrated systems, modular and turn-key
- Training and Education – available onsite or on demand
- Services –Instrument service and calibration and GMP support
Achieving cleanroom control…
is not an isolated exercise. At Particle Measuring Systems we understand that finding the right partner is key to a less stressful solution, especially for pharmaceutical contamination control. Critical instrumentation used for measurement must be accurate and the data must support the whole process. Using the data, allows our team of experts within the PMS® Advisory Services to align your processes with the regulatory demands of EU GMP Annex 1, ISO 14644-1 / 2, USP 788, USP 797, 21 CFR Part 11 and ISO 21501-4 and more.
Data trending, the assignment of appropriate levels and limits, and then how best to react to an out-of-tolerance event can all be supported by helping you understand the data. Move beyond compliance to ensure environmental control and continuous improvement, accelerating change and innovation.
Our teams are direct in every major region, providing you with peace of mind no matter where your offices are located.