A large proportion of products labeled as sterile are manufactured by aseptic processing rather than terminal sterilization. Because aseptic processing relies on the exclusion of microorganisms from the process stream and the prevention of microorganisms from entering open containers during processing, product bioburden as well as the bioburden of the manufacturing environment are important factors governing the risk of unacceptable microbial contamination. The terms aseptic and sterile are not synonymous. “Sterile” is a medieval word derived from the Latin sterilis (unfruitful), meaning, in modern terms, free from living germs or viable microorganisms that have the potential to reproduce. In contemporary aseptic healthcare product manufacturing, “aseptic” describes the process for handling sterilized materials in a controlled environment designed to maintain microbial contamination at levels known to present minimal risk.1 Therefore, the importance of adequate and effective microbiological controls cannot be overstated enough.
Environmental Monitoring (EM) in Pharmaceutical Manufacturing Facilities
Control of the environment in which pharmaceutical products are manufactured is a key element of Good Manufacturing Practices (GMP). Included in this control, the monitoring of microbial contamination is essential in the pharmaceutical, cosmetic, and food and beverage industries.3 Physical and microbial monitoring of manufacturing cleanrooms, Restricted-Access Barrier Systems (RABS), and isolators should include the components listed in this paper. This table in the paper summarizes major components of an EM program. It is important to understand these components, which will help in the development and implementation of an effective and robust EM program. Evaluating the quality of air, surfaces, personnel garments and behaviors in a cleanroom environment should start with a well-defined written program employing scientifically sound methods of sampling, testing, and data analysis.4
This paper also covers
-
Compressed Gases: A Brief Regulatory Overview
-
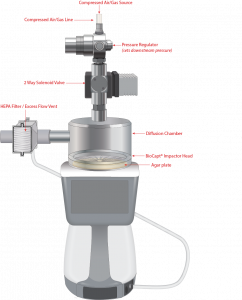
Microbial Impactors with Compressed Gases Kit: How They Work
-
Instrumentation and Selection Criteria
-
Instrumentation Financials and Return of Investment (ROI)
Learn more… Complete the form to download the full paper.