Your Guide to EU GMP Annex 1 - 2022 Environmental Monitoring Regulations
Annex 1 Insights & Updates
The European Commission released its long-awaited Annex 1 regulation in August 2022. Our experts have reviewed this document carefully and have shared their insights with you.
In our first educational webinar since the release of Annex 1 2022, PMS has put two of our experts to the task of explaining the environmental monitoring requirements, providing practical solutions, and answering your questions about the finalized EU GMP Annex 1 regulation. If you missed the popular EU GMP Annex 1 webinar, watch it now on demand.
Read our latest Annex 1 Application Notes by clicking the title below:
Review of Annex 1: 2022
Annex 1 2022 vs. Annex 1 2008: A side-by-side comparison
Validation and Qualification Approach Outlined in Latest Annex 1 Revision
We have many more Annex 1 educational resources


Below are more videos from the experts at Particle Measuring Systems covering Annex 1:
- Update: Continuous Viable Monitoring
- Alternative Micro Methods and Rapid Microbial
- Single-Use Considerations and a Solution
- Cleaning & Disinfection: What you need to know
- Classification – Qualification – Monitoring
- Quality Risk Management (QRM)
- Using a Risk Assessment to set microbiological plate incubation conditions
- Microbiological Plate Incubation
- Data Trending-Alert/Action Level Settings
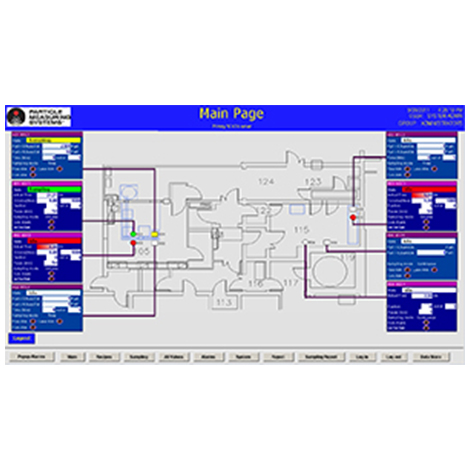
Scroll down for even more of our Annex 1 compliant viable nonviable instruments and data management solutions.
2022 EU GMP Annex 1 Release
In this interview, Mark Hallworth, Sr. GMP Scientist, Particle Measuring Systems, discusses the new EU GMP Annex 1 release. Topics discussed include Annex 1’s history, timing, changes, and next steps.