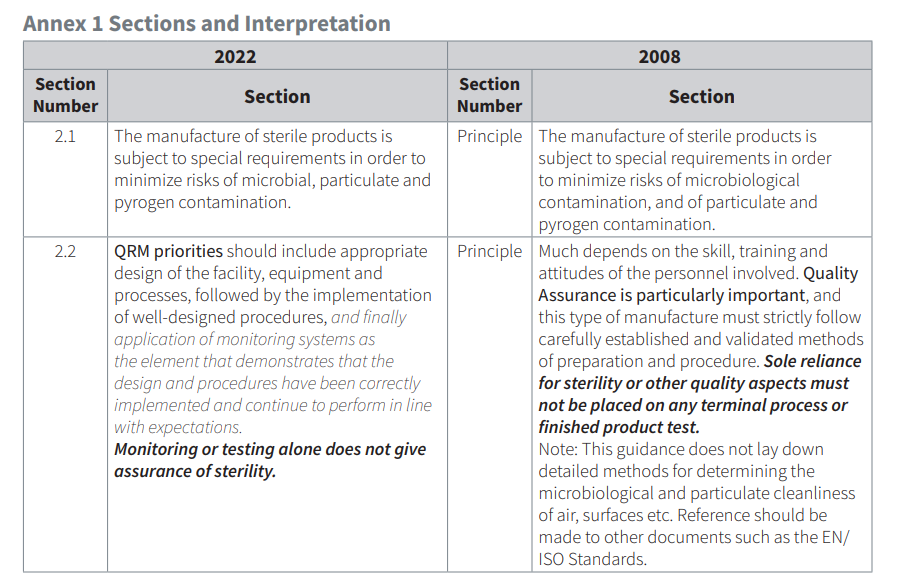
Annex 1 2008 is the basis for the 2022 version. This paper provides an easy-to-understand side by side comparison of the Annex 1 2008 v. 2022 versions, broken down by section.

In August 2022, a new revision of the EU GMP Annex 1 regulatory standard for sterile drug products was released, replacing the most recent draft from 2020 and the existing revision from 2008. The deadline for operational use of the new standards is August 25, 2023: a year after the release. These requirements regulate the manufacturing of sterile drugs made in and imported to the EU. Pharmaceutical manufacturing is performed in controlled environments to reduce contamination, and changes recently announced by Annex 1 focus more on strategic control than on measurement of quality. This new revision also better aligns the manufacturing principles contained in the Annex 1 to those presented by the World Health Organization (WHO), Pharmaceutical Inspection Cooperation Scheme (PIC/S), and US Food and Drug Administration (FDA).
The new revision is a complete rewrite of the existing Annex 1 from 2008 and almost quadruples the length. It divides the document into 10 newly defined sections. One major sectioning change is the separation and differentiation of Certification (Section 4) and Monitoring (Section 9), which allows for expanded guidance and distinction between premise design/qualification and ongoing routine monitoring. There is a new section that discusses the concept of contamination control strategy (CCS). This section shifts to a new paradigm of incorporating CCS as a central holistic approach to how each aspect of contamination interacts with the facility as a whole. There is also a new section that discusses and identifies Quality Risk Management (QRM) as a central principle to defining processes, operations, and limits, and it ties to CCS to balance process against risk. Additionally, as laid out in the new revision, regulations for Environmental Monitoring is essentially the same with a few enhanced descriptions to better align with QRM.
Learn more… Complete the form to download the full paper.