Cleanroom validation and qualification are critical for effective contamination control.
In August 2022, a new EU GMP Annex 1 revision was issued, proposing new regulations for sterile drug products and production. This 2022 release replaces the most recent draft from 2020 and the existing Annex 1 2008. The updated revision further emphasizes validation and qualification topics. In the new Annex 1 better detail about specific needs and requirements for premises, equipment, utilities, and personnel are described to provide guidance for the manufacturing of sterile products.
Why are cleanroom validation and qualification so important in a pharmaceutical process?
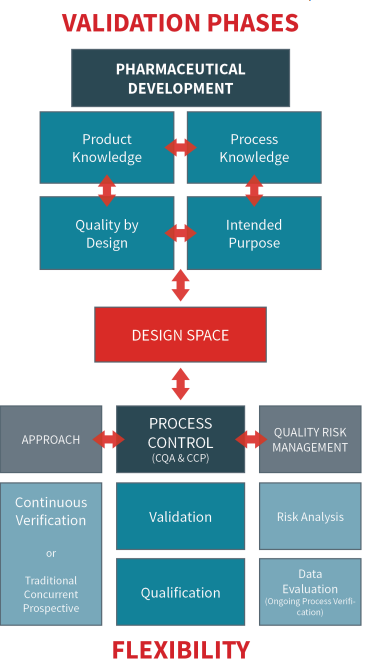 With the inclusion of cleanroom validation and qualification in the flexible and scientifically sound pharmaceutical processes proposed by the 2022 Annex 1 revision, an overall review of pharmaceutical development is essential. The aim of pharmaceutical development is to design a quality product and a manufacturing process to consistently deliver the intended performance of the final product. The information and knowledge gained from manufacturing experience and pharmaceutical development studies provide scientific understanding to support the establishment of the design space – a multidimensional combination and interaction of input variables (e.g., material attributes and process parameters that have been demonstrated to provide assurance of quality).
With the inclusion of cleanroom validation and qualification in the flexible and scientifically sound pharmaceutical processes proposed by the 2022 Annex 1 revision, an overall review of pharmaceutical development is essential. The aim of pharmaceutical development is to design a quality product and a manufacturing process to consistently deliver the intended performance of the final product. The information and knowledge gained from manufacturing experience and pharmaceutical development studies provide scientific understanding to support the establishment of the design space – a multidimensional combination and interaction of input variables (e.g., material attributes and process parameters that have been demonstrated to provide assurance of quality).
Working within the design space is not considered to be a change. Movement outside the design space is a change and would normally initiate a regulatory post-approval change process. Process development studies should provide the basis for any process control requirements. Similarly, process improvements should be supported by several quality aspects including equipment qualification and process validation (or continuous process verification, an alternative approach in which manufacturing process performance is continuously monitored and evaluated, where applicable).
QRM (Quality risk management) should be considered an important tool in overall manufacturing process control. When QRM principles are applied to validation, it supports the robustness of the process in terms of reproducibility within established specifications. The addition of QRM principles effectively incorporates scientific rationales and a risk assessment as critical aspects for considering the raw data and for considering the quality of the product for the final customer. Qualification, as a part of process validation, creates data on critical systems (e.g., equipment, personnel, materials…) that can be used to evaluate the process and document its quality over time (and during routine usage). Installation, operational, and performance evidence for a piece of equipment or system guarantees that the user requirement for assuring product quality for an intended purpose is achieved.
The application of a systematic quality by design approach to development and the application of a quality risk management approach to the manufacturing process (linked to an appropriate pharmaceutical quality system) both generate opportunities to enhance science- and risk-based regulatory methods. A better understanding of the product and its manufacturing process (systematic evaluation) can create a basis for more flexible approaches.
Learn more… Complete the form to download the full paper.