1. Start with this USP 788 overview paper
This paper discusses the requirements laid out by US (USP), European (EP), and Japanese (JP) Pharmacopoeia standards and includes the most recent USP 788 (USP 42 2018), EP 5.1, and JP 17 release information. These standards demand that injectable solutions are effectively monitored for microcontamination, specifically non-soluble particulates.

2. Watch this On-Demand webinar for an overview of the requirements and a solution
USP 788 Requirements & Solutions
Other Webinars to consider:
Meeting USP 729 / USP 788-1788 Regulatory Requirements
USP 1788 2022 Revision Overview
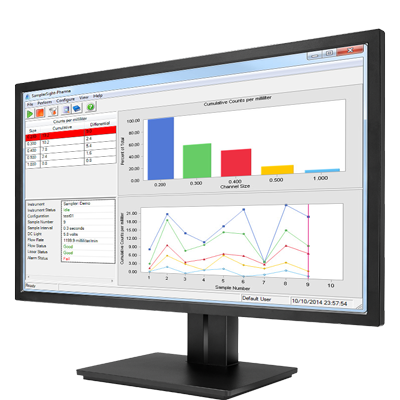
3.Get an effective and reliable USP 788 solution
The APSS-2000 Liquid Particle Counter meets USP 788; sizes & counts suspended particles in a wide range of liquids, including parenterals.

Particle Measuring Systems is direct in every major market and able to ensure the same ongoing support
Liquid Particle Counting Sample Testing Solution
Are you sending out your samples for liquid particle count testing? Heather Mason discusses a money saving solution that also significantly reduces turnaround time. This particle monitoring solution from Particle Measuring Systems meets #USP788, #USP789, #21cfrpart11, and all #Pharmacopia. Only the APSS-2000 uses a particle counting spectrometer to choose multiple particle sizes, allowing for a wide variety of contamination control and sample testing applications.
Learn More