For Aseptic Environment Monitoring, a risk assessment helps to meet the expectations of auditors.
Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards. The risk assessment of particle and microbiological contamination of the product and its components should take into account the following aspects:
- Highest risk of contamination of the product and its components (study of the airflows and exhaust grid)
- Personnel operations
- Material and personnel flows
- Cleaning and sanitizing of environments and equipment.
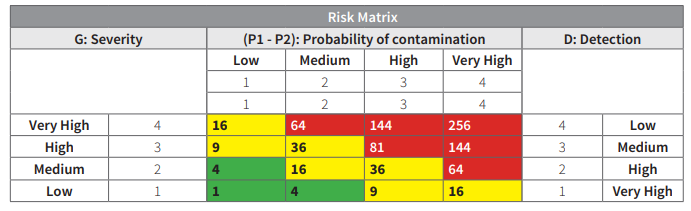
Two clean areas are of particular importance to sterile drug product quality: the critical area and the supporting clean areas associated with it. A critical area is one in which the sterilized drug product, containers, and closures are exposed to environmental conditions that must be designed to maintain product sterility. Activities conducted in such areas include manipulations (e.g., aseptic connections, sterile ingredient additions) of sterile materials prior to and
during filling and closing operations.
Areas become critical when ….
Learn more… Complete the form to download the full paper.