Analysis of an Aseptic Process and Defining a Cleaning and Sanitization Plan
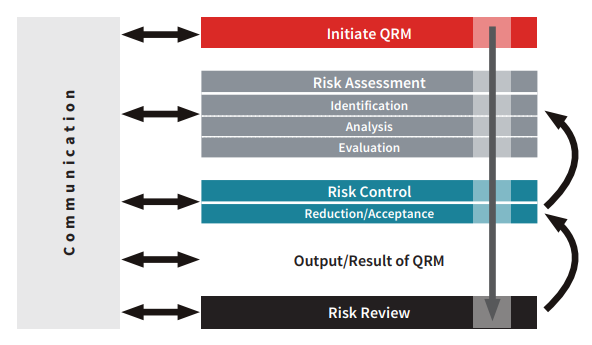 Cleaning and disinfection plans are a complex strategy to manage contamination risk in classified areas. Production residues (powder, oil, salts, etc.) and personnel-related contamination risk (production line in isolator, RABs, open line) should be evaluated first before preparing and implementing cleaning and disinfection plans. The key aspects of decontamination should be defined according to the risk management approach. Variables that increase or decrease risk should be identified, analyzed and assessed in order to develop, maintain and improve process control.
Cleaning and disinfection plans are a complex strategy to manage contamination risk in classified areas. Production residues (powder, oil, salts, etc.) and personnel-related contamination risk (production line in isolator, RABs, open line) should be evaluated first before preparing and implementing cleaning and disinfection plans. The key aspects of decontamination should be defined according to the risk management approach. Variables that increase or decrease risk should be identified, analyzed and assessed in order to develop, maintain and improve process control.
 Even industry-leading monitoring instruments and procedures are not able to guarantee that all living species within an environment will be found for identification. Different varieties of microorganisms are seen throughout many manufacturing environments, making it important to identify those that are relevant to an individual process. This is a difficult task, as many microbes do not grow on standardized media. In particular, detection of biofilm, a group of microorganism cells stuck together, is one of the greatest challenges. Aside from that which is required for metabolism, biofilm is not permeable to external substances, making it also difficult to remove. Alert and action limits for contamination monitoring should be established for process analysis and control. Any variability in monitoring data should be analyzed in order to prevent serious contamination risk, and alert levels should adapt to changing trends.
Even industry-leading monitoring instruments and procedures are not able to guarantee that all living species within an environment will be found for identification. Different varieties of microorganisms are seen throughout many manufacturing environments, making it important to identify those that are relevant to an individual process. This is a difficult task, as many microbes do not grow on standardized media. In particular, detection of biofilm, a group of microorganism cells stuck together, is one of the greatest challenges. Aside from that which is required for metabolism, biofilm is not permeable to external substances, making it also difficult to remove. Alert and action limits for contamination monitoring should be established for process analysis and control. Any variability in monitoring data should be analyzed in order to prevent serious contamination risk, and alert levels should adapt to changing trends.
GMP guidelines request that for each action limit excursion, all recovered isolates (all microorganisms detected in aseptic areas) must be identified in order to understand the root cause of contamination.
COMPELTE THIS FORM TO LEARN MORE
