Microbial Impactor: BioCapt® Stainless Steel
Sensitivity: 1 cfu
Discover the BioCapt® Remote Microbial Impactor
Particle Measuring Systems engineers, manufactures, installs, calibrates, repairs, and maintains the BioCapt® Remote Microbial Impactor.
Product Details
- Stainless steel
- Protects petri dish from unintentional contamination
- Vacuum draws air down over media for viable particle sample collection
- Meets ISO 14698-1/Annex B for physical and biological collection efficiencies
- 316L stainless steel atrium is autoclavable
- Optimal slit design ensures microorganisms are undamaged during sampling
- Immediate and easy detection of false-positives
- Viable microbial air sampling in cleanrooms and associated controlled environments
- USP 797 environmental viable-airborne particle testing
- Bioburden monitoring of medical device manufacturing environments
- Microbial monitoring of aseptic manufacturing areas
Resources
- Environmental Monitoring in the Pharmaceutical Industry: Handbook
- Regulatory Compliance with Pharmaceutical Viable Air Monitoring in ISO 5 (Grade A)
- Microbial Control and Monitoring in Aseptic Processing Cleanrooms
- Microbial Data Collection: Managing the Expectations of Auditors
- Microbial Monitoring of Compressed Gas
- Microbial Survival in Compressed Gases Under Fast Decompression to Normal Atmospheric Conditions
- A Review of EN ISO 17141, ISO 14698 andInstrumentation Conformance
- Quality Risk Management: Does QRM + RMM = Better EM?
- Environmental Monitoring Data Quality: A New Challenge
- Comparing Microbiological Air Monitoring Techniques for Critical Environments
- Designing an Environmental Monitoring Solution for GMP Applications
- Alarm Rationale for Continuous Particle Counting Systems
- Cleanroom Classification for Pharmaceutical Applications
- How to Build a Contamination Control Strategy: Considerations and Steps
- Active Air Sampler Comparison in Remote Settings
- Particle Data Collection and Interpretation for ISO Cleanrooms
- Feller Table of Statistical Corrections for BioCapt® Instruments
Accessories
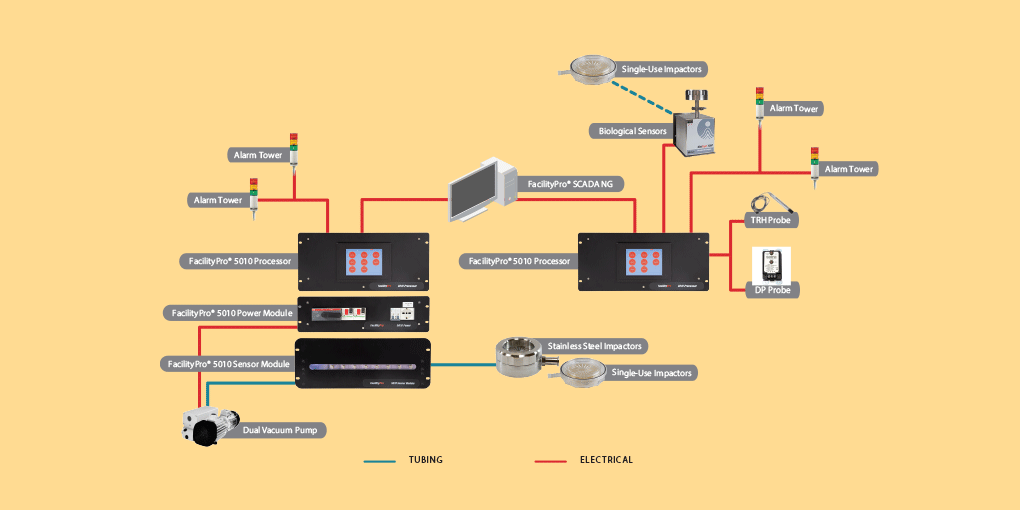
Part of a Facility Monitoring System
Add the BioCapt stainless steel to your Facility Monitoring System.
Not sure where to start? Here are the next steps:

Tell us about your application requirements.

Our experts will help you find the right solutions to meet your specific requirements

Once we identify the best solution for you, we provide you with pricing and delivery dates.
Related Products











