Pharmaceutical products are manufactured to meet exacting standards of both efficacy and quality. All aspects of quality are reviewed considering the risks associated with the delivery method (injected, ingested etc.) and the manner in which they were produced (aseptic, terminally sterilized, or under lesser controlled conditions). This paper looks at two parts of that process: the quality of the environment in which the product is manufactured and the standards that surround the particle concentration limits that determine what a controlled environment consists of.
This paper examines the standards for physical testing (EN ISO 14644-1:2015) and those standards which apply in regulatory guidance (EU GMP Annex 1).
In 1999, the new ISO 14644 room classification suite of standards became active, the first of which was ISO 14644-1, which determined the method by which a room should be classified and the maximum allowable particles within a fixed volume of air. The reader should note that although ISO 14644 has been adopted globally for cleanroom classification, there are differences for routine monitoring, particularly between ISO 14644 and EU and WHO GMP.
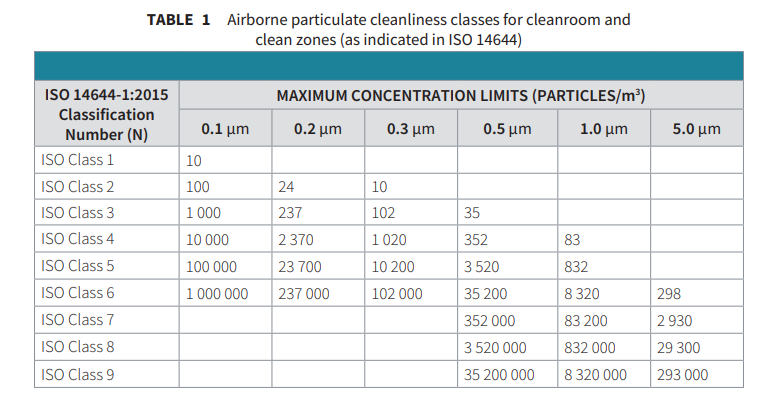
The certification state of the cleanroom must be defined in advance of testing; three states exist within the context of ISO 14644-1:
As Built: a completed room with all services connected and functional but without production
equipment or personnel within the facility.
At Rest: all the services are connected, all the equipment is installed and operating to an agreed
manner, but no personnel are present.
Operational: all equipment is installed and is functioning to an agreed format and a specified number
of personnel are present, working to an agreed procedure.
Complete the form to download the full paper…







