All pharmaceutical and biotech products must be manufactured in accordance with the current Good Manufacturing Practice (cGMP) regulations. Environmental monitoring of these pharmaceutical manufacturing areas meets the requirement for contamination control of an environment, which is essential in defining clean manufacturing and demonstrating the necessary controls are working. A pharmaceutical company must have a quality department that is responsible for the routine quality assurances that:
Establishes documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.
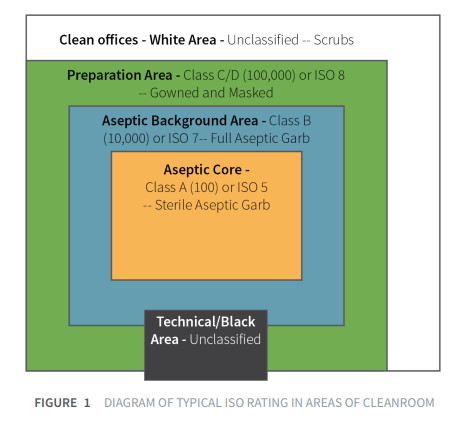
To satisfy these requirements, the products are manufactured in controlled environments/cleanrooms. Cleanrooms are employed to reduce the variability of production environments, and as controlled environments they can be tested and proven to meet specific standards. GMP require that these environments be rigorously tested and monitored to ensure that there is full and constant awareness of current environmental conditions for both viable and nonviable contamination.
A cleanroom is the fundamental starting point for contamination control; a cleanroom is defined as:
A room in which air filtration, air distribution, utilities, materials of construction, and equipment are maintained in a controlled manner.
Operational procedures are defined and regulated for airborne particle concentrations to meet appropriate particulate cleanliness classifications. The International Standard, ISO 14644-1 is the current international standard of defining cleanroom contamination levels.
Pharmaceutical cleanrooms are classified according to required particle concentrations of the air that meet the cleanliness criteria for the manufacturing process being performed. The determination of the cleanroom class is a process based on statistically valid measurements, and its a function of the filtration and operations status of the room; it is, in essence, a calibration of the room to ensure it meets its intended classification. It is not, primarily, a function of risk of application.
Complete the form to download the full paper…






