Cleanroom Design is the basis for contamination management.
To satisfy the assurances required by regulatory agencies, pharmaceutical products are manufactured in a controlled environment. Cleanrooms are an example of a controlled environment and are employed to reduce the contamination risk and variability of potential production environment. As controlled environments, cleanrooms can be regulated to meet specific standards. GMP regulations require that these environments are rigorously monitored to ensure that there is full and constant awareness of current environmental conditions for both viable and nonviable contamination.
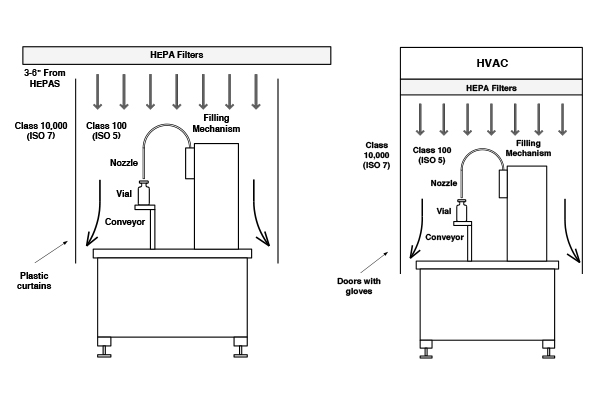
A cleanroom is the fundamental starting point for contamination control. A cleanroom is defined as a room in which air filtration, air distribution, utilities, materials of construction, and equipment are maintained in a controlled manner. Operational procedures are defined and regulated for airborne particle concentrations to meet appropriate particulate cleanliness classifications.
The nature of activities and choice of a cleanroom design affects the type of environmental monitoring required. The higher the access of operators to a process, the greater the risk of contamination. This is because personnel are the single largest contributor of airborne contaminants within a cleanroom.
There are essentially two basic types of cleanrooms:
- Turbulent dilution of contamination
- Unidirectional Air wash
Complete the form to download the full paper…