How to Build a Contamination Control Strategy for Pharma Manufacturing
When considering how to build a contamination control strategy, we need to start with context. Mass-produced pharmaceuticals have come far since the industrial manufacturing of synthetic drugs at the end of the 19th century with rapid advancement in technology, process control, and sterility requirements. Yet even as improvements to Pharma develop, recalls still occur with regularity and their significant impact on both the manufacturer and consumer are why worldwide regulation of the industry is so enforced. Environmental monitoring (EM) is one facet of the greater picture of current good manufacturing practices (cGMPs) that has been standardized for the safety of human health.
In a typical cleanroom, EM takes the form of monitoring non-viable and viable particles to maintain a reliable state of control in production. Limits per an area’s classification or “grade” are defined by both the FDA’s Sterile Drug Products Produced by Aseptic Processing, and the European Commission’s EU GMP Annex 1 2020 Draft.
- Viable particles are living organisms such as bacteria, molds, and yeasts.
- EU GMP limits found in Table 7, Maximum action limits for viable particle contamination.
- FDA cGMP limits found in Table 1, Air classifications.
- Nonviable particles do not contain a living organism, but act as transportation for viable particles.
- EU GMP limits found in, Table 6, Limits for airborne particulate concentration for the monitoring of non-viable contamination.
- FDA cGMP limits found in Table 1, Air classifications.
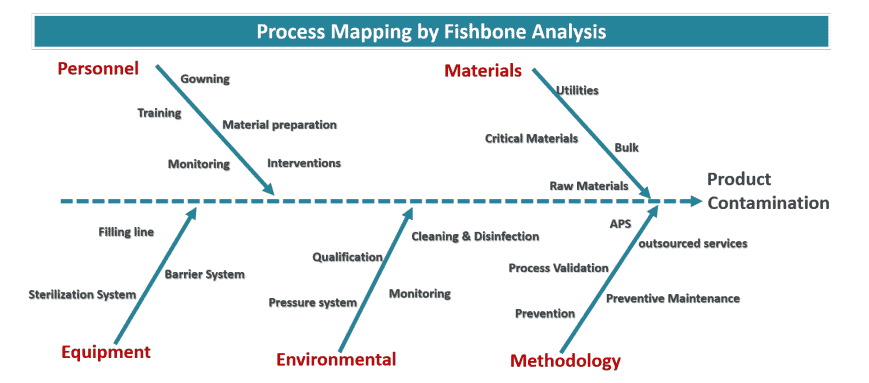
Past focus was placed on sampling methods for the measurement of potential contamination: particle counters, active air samplers, Petri dishes, and swabs for surface monitoring. Bioburden of bulk product and filter integrity were also tested to increase the detection of microbiological contamination along the production process. This approach has been remodeled to prevent and control potential contamination from reaching the point of no return: the product. Once contamination occurs, there is no cost-effective removal scheme to continue production, making the determination of sources all the more important. This is achieved by:
- Implementing Quality by Design into the manufacturing area.
- Using quality risk management to identify the critical control points (CCPs).
- With the CCPs, identifying the process phases where it is necessary to establish mitigation actions to control the identified risks.
The collective execution of controls is called the contamination control strategy (CCS).
Also in this paper
- Defining Contamination Control Strategy (CCS)
- Finding Root Causes of Contamination
- Designing a Cleanroom
- ….Much more
This paper was made in conjunction with the webinar, “Considerations and Steps for Building a Contamination Control Strategy” See an FAQ document here. Many thoughtful questions were asked about non-viable particle counting requirements in cleanrooms and sampling placement pertaining to process design. 
Complete the form to download the full paper…