With the release of the new EU GMP Annex 1, a review of current practices is required to ensure that the installed monitoring system, chosen to meet the needs of Annex 1, complies with the requirements. This chapter will review the needs of Annex 1 with systems designs currently being installed.
Cleanroom Classification
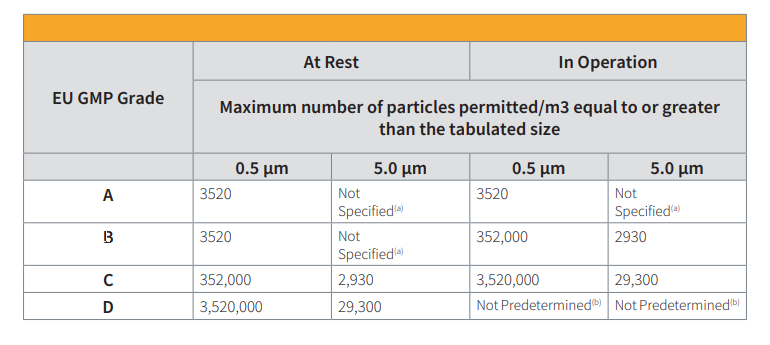
Pharmaceutical cleanrooms are classified according to the particle concentration of the air that is required to meet the cleanliness criteria for the manufacturing process being performed. The determination of the cleanroom class is a process based on actual statistically valid measurements, and a function of the filtration and operations status of the room, it is, in essence, a calibration of the room to ensure it meets its intended classification, it is not, primarily, a function of risk of application.
There are three measurement phases involving particle counting in cleanrooms:
As Built: a completed room with all services connected and functional but without production equipment or personnel within the facility.
At Rest: all the services are connected, all the equipment is installed and operating to an agreed manner, but no personnel are present.
Operational: all equipment is installed and is functioning to an agreed format and a specified number of personnel are present, working to an agreed procedure.
The airborne particle count test is performed by counting particles at defined grid locations within the cleanroom. The test points should be equally spaced throughout the room and at work height to demonstrate the quality of the air cleanliness at the work area. Pharmaceutical cleanrooms typically operate at Class 5 (most aseptic areas), Class 7 (surrounding areas), or Class 8 (support areas). See Figure 1 on the following page for a visual representation.
Complete the form to download the full paper…






