Environmental monitoring trend analysis for trends at an appropriate frequency is essential to aid in the interpretation of process stability and assess overall environmental control performance.
Appropriate alert and action limits should be set for the results of particulate and microbiological monitoring. It’s possible to use the limits reported in FDA guidelines or Annex 1 of Eudralex – Volume 4 when the facility is new and/or there is not available data. When there is statistically significant data, it’s essential to establish the proper alert and action limits.
Statistical methods are intended to improve the quality of decision making. The methods may be used in an ongoing program to analyze collected data. Timely evaluation of data allows the prompt detection of undesired process variation, which facilitates process understanding and may mitigate response variability. The case study discussed in this paper involves the use of trend analysis to analyze microbiological and total particle monitoring data and statistical analysis to define alert and action limits for grade A, B, C and D.
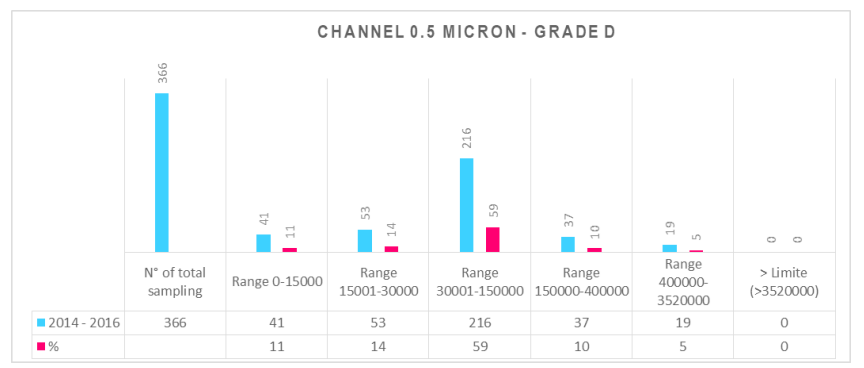
Analysis trends are useful in detecting patterns that could lead to future quality problems, and in anticipating future performance. In the interpretation of GMP requirements, regulatory authorities increasingly request an assessment of trend inspections. The prompt detection and evaluation of trends supports the implementation of corrective and preventive actions (CAPA) as suggested by ICH Q10.
Statistical methods allow for the control of contamination as a result of understanding trends and changes in data. In the case of positive or negative trends, it’s necessary to do an investigation to trace the causes of change. The alert and action limits are statistically derived, and are used to define the typical operating range for the process.
Once the limits are statistically established, it is necessary to define, in a standard operating procedure, the steps to take in case of out-of-alert or -action limits. Prompt recognition of deviations from typical performance enables a careful and complete process review, and the ability to improve the process.