ISO 14644-1/2: 2015 Cleanrooms and Associated Controlled Environments Insights & Solutions

ISO 14644-1/2 Education and Resources
ISO 14644-1/2:2015 continues to pose challenges to cleanroom managers and manufacturers. Here we provide several sources of information to help everyone from those new to this ISO regulation to veterans in the field.
Our team of regulatory experts has put together a package of information so you can easily understand the requirements and what you need to do to meet them. Get a summary of ISO 14644-1/2:2015 by reading this paper or watching the on-demand webinar here. We also have FAQs and a paper to learn about the closely relevant ISO 21501-4 calibration standards and how they apply to you.
Do you need an ISO 14644-1/2:2015 compliant product that is supported with ISO 21501-4 calibration?
PMS has even more services to support your ISO 14644 contamination control efforts:
- Our Contamination Control Advisory Services can conduct a Risk Assessment for your pharmaceutical processes
- PMS Cleanroom particle counters: including portable/mobile and fixed/remote
- Microbial Monitors: including portable/mobile, fixed/remote, and single use.
- Data management software from Particle Measuring Systems
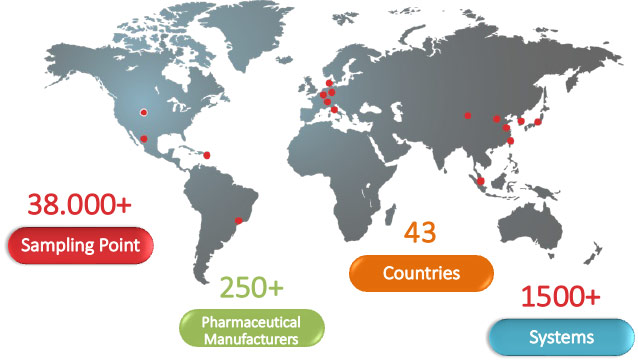
Particle Measuring Systems is direct in every major market and able to ensure the same ongoing support no matter where you are located. Contact us today for a quote.
Understanding ISO 14644-2 Cleanroom Monitoring
Over the last several years, ISO has been working on the revision of the basic airborne cleanliness classification, 14644-1 and -2. The ISO community voted in favor of the revision to update and improve the standard specifically to:
- Simplify the classification process
- Review the classification procedure and make it more applicable to cleanroom operation.
These changes are being made to update the standard to current thinking and industry requirements, and to avoid any radical change to the principles of the current ISO cleanliness classes 1-9.
Learn more about the revision and cleanroom monitoring according to ISO 14644 from this on-demand webinar.