The Continuous Monitoring Concept for Pharmaceutical Manufacturing
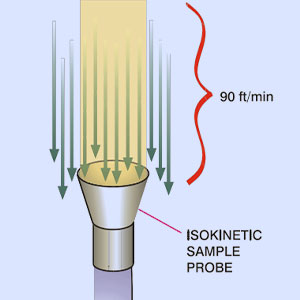
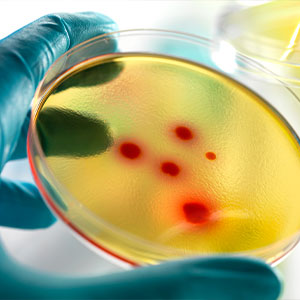
Continuous monitoring helps ensure the sterility of clean manufacturing within the pharmaceutical industry. In the pharmaceutical field, one of the main objectives required by regulatory guidelines is to preserve the sterility of cleanrooms. Cleanrooms are controlled areas where contamination levels are monitored and managed to meet a defined cleanliness level. To reach this goal, it is important to ensure that each GMP requirement is respected over time.