The primary importance of environmental monitoring for Advanced Therapeutic Medicinal Products (ATMP) facilities is to demonstrate the highest degree of control over
- The aseptic manufacturing environment
- The potential for cross contamination between batches / manipulations
Therefore, the manufacturing facility should have a comprehensive environmental monitoring program, which includes monitoring for nonviable airborne particles, viable airborne particulates, and surface viable contamination, in the aseptic areas. These procedures should address frequencies and locations for the monitoring sample points, warning, and alarm limits for each area, and corrective actions which need to be undertaken if any of the areas show a deviation from expected results.
A Contamination Control Strategy (CCS) will include the environmental monitoring program and should be implemented across the facility. The CCS should define critical control points as part of a risk assessment and assess the effectiveness of the controls and monitoring measures used to manage risks associated with contamination.
One aspect of the system is the location of the sample point, these should be determined following a documented Environmental Monitoring Risk Assessment (EMRA) and include the following information: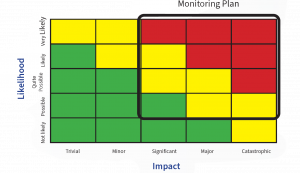
- Sampling locations
- Frequency of monitoring
- Monitoring method used and
- Incubation conditions (e.g. time, temperature(s), aerobic and/or anaerobic conditions).
The risk assessment is based on inputs from the different groups within the facility.
Typical Continuous Monitoring System
Instrumentation used in constructing an integrated solution will typically include:
Particle Counting – The need for continuous data requires a dedicated sensor at each location that samples continuously during the set-up and production phases of manufacturing. Sample points will be mounted within the hood or isolator line and connected to the sensor via a short length of sample tubing (no more than 2 m, ideally shorter). The location and orientation of the probe are dependent on the findings of the risk assessment.
Microbial Sampling – Where a risk has identified the need for total particle counting, there is an associated requirement for microbial sampling.
Active Air Sampling – Only the sample head is placed within the environment, ensuring that any sample is not exhausted locally within the critical space. The sampling is quantitative and can run continuously for up to 4 hours*. Start and stop controls are performed via the software interface.
Static/Passive Air Sampling – Here, a plate is placed in the local environment for a period of up to 4 hours and is an additional valuable data point in the overall understanding of the microbial risk. The active nature of impaction sampling can damage certain microbiology, and although this technique does not need to be part of routine monitoring, it should form an aspect of qualification studies and the EMPQ.
*see this paper for more information
Learn more… Complete the form to download the full paper.






