Vaporized Hydrogen Peroxide (VHP) Resistance Testing for MiniCapt Pro Microbial Sampler
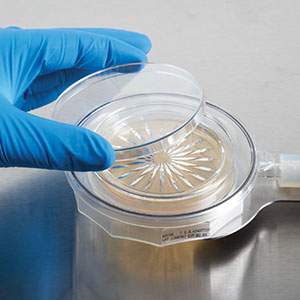
Vaporized Hydrogen Peroxide (VHP) serves as a potent oxidizer and has long been acknowledged for its effectiveness in sterilization within enclosed filling isolators. With the rising prevalence of isolator applications, there’s an increasing demand for robust samplers capable of enduring such environments. The MiniCapt® Pro Remote Microbial Sampler features an internal flow path designed to resist the corrosive impacts of VHP.