What Data Management Factors Should be Considered When Setting up Environmental Monitoring in a Pharmaceutical Manufacturing Facility?
Pharmaceutical Manufacturing Data Managment is one of the important steps for effective contamination control In a typical pharmaceutical manufacturing plant, there are several important tasks to ensure the product can be handled without being contaminated. All these steps must be documented to ensure \every person did what they were supposed to do. In the GMP world, we have the principle, “If it is not documented, it did not happen.” This documentation is often done on paper, which results in information in many different databases and sometimes even paper files that are not easily accessible. Because the documentation is not accessible, it is hard to take the data into consideration when the people responsible for environmental monitoring for a pharmaceutical manufacturing facility have to assess the current situation and plan for the future. This process of future planning is called trending, and it is nearly impossible on paper.
Regarding Pharmaceutical Manufacturing Data Managment, the important data, which is used to confirm that the manufacturing was done as planned, must be compiled and verified. This process often leads to thousands of pages of printed-out documents. People have to compare the data from different sources, and this process is time-consuming, often needing many hours or sometimes days. Due to the quantity of data, it is not possible to do this on paper anymore in a mid-size pharma company. This is why current regulations expect the pharma manufacturers to do this trending in a computerized system.
Pharmaceutical manufacturers are challenged to get this data into a digital format. An extreme example is the mobile particle counter which measures nonviable particles in a pharmaceutical manufacturing clean room. Here, the data is created in an electronic instrument, printed out, and photocopied for digitalization; then, the paper copy and scanned digital copy are stored. Because the digital data is lost when the instrument creates the printout, someone has to type in the data from the paper printout into Excel for further trending leading to the storage of the same data in a different format. We see typically at least seven different places where important environmental monitoring data is being stored. For effective trending, all this data from all these databases must be combined manually by retyping it into another software.
Particle Measuring Systems (PMS) has partnered with Novatek who started with environmental monitoring Software in 2003. They used to handle mainly manually collected data which was previously written down on paper. Novatek developed software to handle all this data in a central, paperless database. They worked with hundreds of different manufacturers on how best to read and present the data to Authorities and created software that generates all the reports needed for pharmaceutical manufacturing sites around the world.
PMS combined the knowledge of Novatek with the knowledge of collecting automatically created data from sensors in cleanrooms. These sensors feed the data into a central computer system, and from there, the data is combined with the environmental monitoring software, PharmaIntegrity. The PharmaIntegrity software has the ability to handle and trend data from all major areas that are important to pharmaceutical environmental monitoring systems. PharmaIntegrity can take the data from remote, online, and/or mobile particle counters and microbial collectors. It can take online data from the room sensors like humidity, temperature, differential pressure, and/or airflow sensors. It is possible to connect other sensors from utility monitoring or final batch release data from a sterility test of a subvisible particle counter. Any sensor with a 4-20mA output can typically be integrated with no extra effort.
The largest quantity of data comes from manual microbial testing like fingerprinting and plating of operators after production and from surface monitoring. After incubation, the microbial counting and identification can be entered directly by the laboratory. The people who review the data can see the result in real-time.
The approval process is also completely done in PharmaIntegrity, and the whole process from collection to approval and through trending is done in the same system. Trending can reach back on data for many years through the whole microbial life of the facility. All other environmental monitoring data points can be viewed at the click of a button.
Also, all the data from all the work that is done to qualify and recertify the cleanrooms is being fed and stored in the system. This is very helpful because the users can always go back to the basic data and compare with it.
Summary
This PharmaIntegrity Environmental Monitoring Software and Systems is a real opportunity to improve processes for all newly built pharma facilities. With the right preparation, all the interfaces can be planned into the new building to make sure that all installed sensors can interface with the software. This way, it is not necessary to implement them at a later time at a higher cost and a harder, more time-consuming installation. Most of the time, if the facility is not planned in the right way, the Environmental Monitoring (EM) operators will go through the pain of manually collecting the data on paper and retyping it into Excel; then, once the decision to switch to a centralized software is made, the production must be interrupted and money and time must be spent on integrating a new system that the engineers did not plan for upfront.
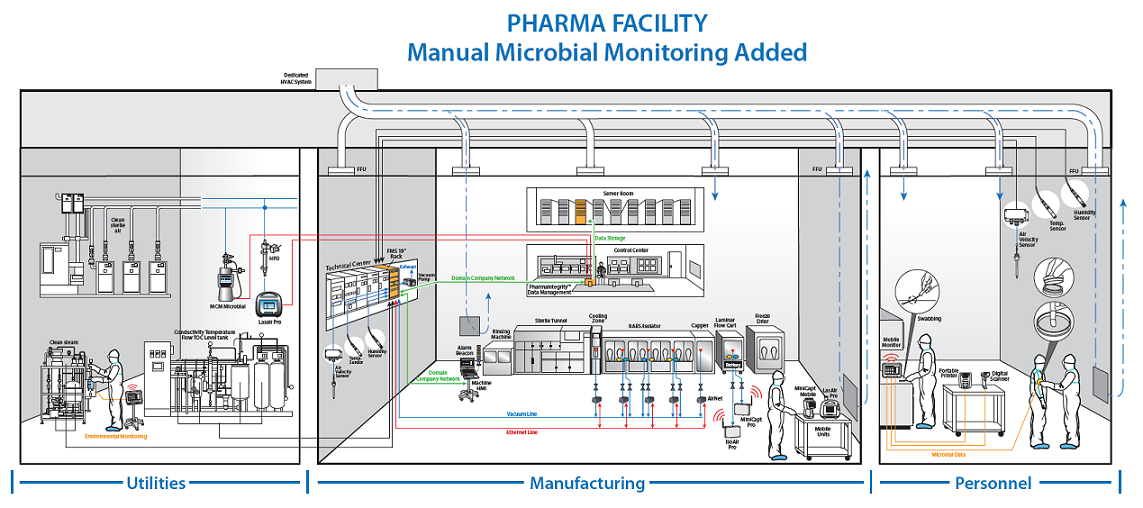
In this drawing, you can see the most important points of environmental monitoring that need to be tracked and trended.
Important note if you are renovating or planning a new pharma facility: If you are planning your facility without an automatic interface for all of these, you are setting your facility up for lots of manual, redundant work. Think about it and your future employees will thank you for it.
Environmental/ Sustainability Note: You can also help to save the environment by avoiding printing thousands of pages of reports a month.
If you have any questions, please contact our team of environmental monitoring artists at PMS.
Learn more about PharmaIntegrity for Pharmaceutical Manufacturing Data Managment.