The new EU GMP Annex 1 specifies the requirements of a pharmaceutical risk assessment to set appropriate microbiological plate conditions. This blog discusses this requirement to use a risk assessment, and then provides an interpretation on how to apply it and use it in different stages of the manufacturing process.
There are three main concepts regarding this:
- “Quality Risk Management” applies to all of Annex 1 and is the underlying concept for understanding and interpreting Annex 1.
- A relevant Pharmaceutical Risk Assessment is expected for each area.
- Companies are expected to implement a Quality Assurance system to fulfill the quality objectives associated with various areas of interest
Section 9 of Annex 1 says that a Risk Assessment should be used to establish a comprehensive environmental monitoring program, including:
- sampling locations
- sampling frequency
- method used
- incubation conditions
A pharmaceutical Risk Assessment should be based on knowledge of:
- historical monitoring data
- monitoring data obtained during qualification
- knowledge of typical microbial flora isolated from the environment.
The result of the Risk Assessment is to establish the most appropriate incubation conditions to identify all potential microorganisms associated with an environment. That is, the goal is to demonstrate that your methods have considered all risks for maximum recovery of microorganisms. This is particularly important in Grade A and B Cleanrooms where the counts are generally very low, and the environmental conditions do not favor microbiological detection.
Next steps
- Establish the most appropriate incubation conditions to identify all potential microorganisms associated with your environment.
- Take information from USP and EU Pharmacopeia
- Risk Assessment: Evaluate the environment and process/product
- Risk Assessment: Review data regularly to confirm the effectiveness of the conditions
A Pharmaceutical Risk Assessment should be holistic and evaluate the characteristics of the process, products, facilities and environmental conditions that favor the growth of:
- anaerobic microorganisms
- slow-growing microorganisms
- yeasts and molds
There are differences in performing a Risk Assessment in existing or new departments. For example, in existing departments, there is historical data would should be considered. In a new department with no historical information, you must define the incubation condition based on a scientific-based approach:
Ready to get started?!
Particle Measuring Systems has a contamination control advisory team that can provide you with sterility assurance audits to prepare you for the review of the regulatory agencies or to identify the areas where to improve.
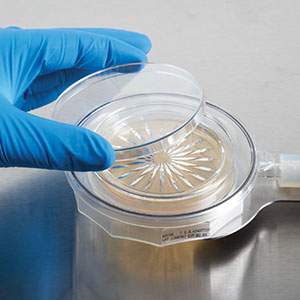 BioCapt® Single-Use Microbial Impactor
BioCapt® Single-Use Microbial Impactor
The BioCapt® Single-Use Microbial Impactor from Particle Measuring Systems eliminates the risk of false positive results and final product contamination from microbial sampling interactions, while decreasing the cost of investigations. The single use sampling head enables manufacturers to monitor their full process, eliminating the risk of non-conclusive qualitative results from settle plates.
The innovative design combines a media plate and sampling head into a single unit. This unique solution eliminates the handling risk, costs, and complications related to autoclaving and stainless-steel head disinfection.


