More than a century ago, Julius R. Petri introduced the Petri plate while collaborating with Robert Koch’s lab. This simple yet iconic device remains widely employed for microbiological environmental monitoring, evolving from early glass versions to the contemporary single-use, sterile Petri plates. Here we will briefly explore the history, applications, and recent advancements in single-use impactors for active air microbial sampling, with a focus on a groundbreaking single-use alternative.
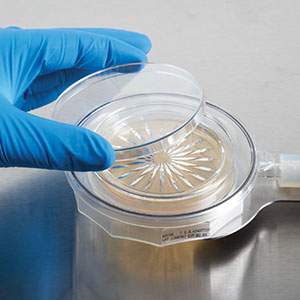
Innovation in Microbial Active Air Sampling: A Novel Single-Use Impactor: A recent breakthrough in microbiological instrumentation introduces a novel, single-use, sterile, transparent plastic impactor integrated with an agar culture media plate. This innovative design serves as an alternative to traditional stainless-steel impactors in environmental monitoring. The single-use microbial device eliminates the need for conventional agar plates and minimizes operator intervention during plate substitution, exposure, and removal.
Airflow Visualization Studies: To validate the effectiveness of this design, airflow visualization studies were conducted. The results show the device’s capability to efficiently capture and retain microbial contaminants, ensuring accurate and reliable results in air monitoring processes.
Validation Performance Data and Benefits in Aseptic Manufacturing: The long-form paper associated with this blog (link below) explores the validation performance data supporting the use of the single-use microbial sampler impactor in microbiological environmental monitoring. The implementation of this device has demonstrated significant benefits. By reducing the risk of operator-induced contamination and streamlining the monitoring process, the single-use impactor contributes to maintaining the sterility of products assembled from sterile components.
Highlights: BioCapt Single-Use has an efficiency performance comparable
to the BioCapt Stainless Steel.
1. The BioCapt Single-Use sampling device has a radial slit impactor
design showing physical and biological efficiency performances
comparable with the BioCapt Stainless Steel sampling head.
2. The leakage test demonstrated that 100% of the air sampled comes
from the air drawn through the slits of the sampling head by the
suction effect of a vacuum pump.
3. The BioCapt Single-Use complies with ISO 14698-1 (Clean rooms and
associated controlled environments – Biocontamination control –
Part 1: General principles and methods (ISO14698-1:2003))
4. The quality of the microbiological ready-to-use culture media used to
fill the BioCapt Single-Use system complies with the corresponding
specific release (Validation study).
