Learn about 21 CFR Part 11 - Electronic Records and Signatures requirements
Webinar: Tackling the 21CFR11 Challenge: From Paper to Paperless
Complying with the new 21 CFR Part 11 requirements present more challenging requirements. Bringing manufacturing processes and controls to a new level can appear simple in theory but provides many challenges in application. Using the example of a standard Data Management implementation, this on-demand webinar presentation provides a variety of insights and solutions.
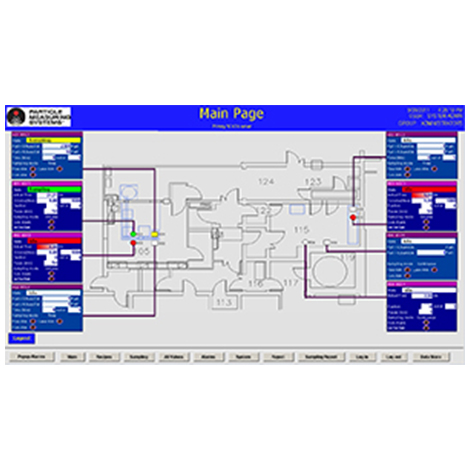
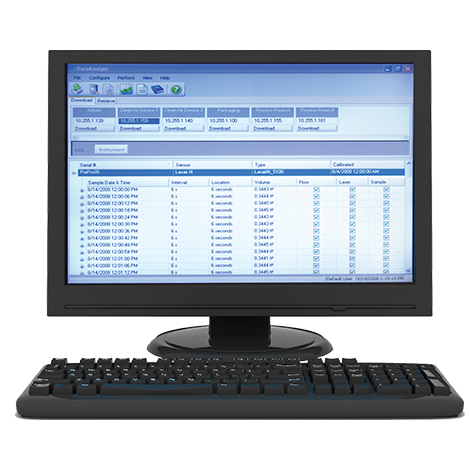
FDA Compliant Data Storage for Compliance and Analytics in Pharmaceutical Manufacturing
This video provides cleanroom environmental monitoring solutions for sampling, reporting, and data retention of particle, microbial and environmental data. The solutions focus on meeting 21 CFR Part 11 data integrity requirements resulting in efficient access to data, and eliminating human error.