In our previous blog in this series, we introduced you to the contamination control strategy (CCS), and why you need one. This time, we look at the quality risk management approach to preparing the CCS in order to find the root cause of your contamination and design the ideal cleanroom for your Pharma process.
See the previous blog in this series here.
Finding the Root Causes of Contamination: Quality Risk Management
Quality By Design (QbD) and Quality Risk Management (QRM) are both inherently linked to process understanding. The steps to achieving a full risk evaluation of a process are outlined step by step.
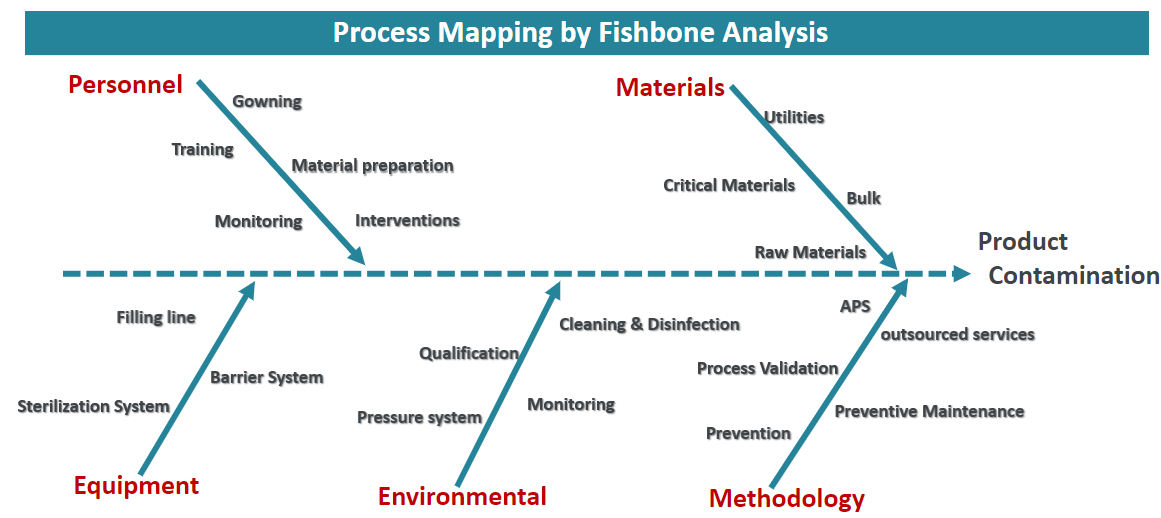
Step 1: Process Mapping
Process mapping allows the risks of particle ingress to be identified. First, a fishbone model is created to document all possible sources of contamination. The root causes can be grouped into representative categories (Personnel, Materials, Equipment, Environmental, and Methodology), but your fishbone may need other categories to account for specialized attributes found in your process. There is no exact standard to follow, which is why process understanding is so essential.
Step 2: Fishbone Analysis
Granularity can be added to fishbone diagrams. Take the example of Utilities within the Materials category. Within this aspect of the process, there are multiple steps that may contribute to contamination. A description of the Water for Injection (WFI) phase within the Utilities area could look similar to:
- Production: Downstream of WFI
- Production: After the storage tank
- Upstream of delivery pumps for the loop
- Distribution of the water inside the loop
- Points of use
- Sampling points
Step 3: Decision Tree
To determine if risks are critical and in need of control, a decision tree specific to the process is created. Here, questions will be tailored to better assign the label of CCP, which indicates control is required to limit contamination. Again, there is no set map of yes or no questions to use—process understanding is key.
Step 4: Assigning Criticality
The HACCP approach to identifying risk is qualitative, meaning the risk is identified but not the associated level of criticality and the real impact on the product. To make the risk determination quantitative, the Failure Modes and Effects Analysis (FMEA) approach is used. The FMEA matrix incorporates a cross of three parameters: Severity, Occurrence, and Detectability, and allows the identification of how critical a risk is to the process.
From here, a control and mitigation plan can be determined based on the level of assigned criticality. CCPs must be evaluated and their risk criticality identified.
Putting It All Together: Cleanroom Design
In order to design a cleanroom according to sterility assurance principles, a risk mitigation plan encompassing set actions and controls must be implemented. This plan could take the form of a redesign, design loop, or a series of valves to reduce contamination. Other possibilities include a system validation or re-validation, and improvements to sampling procedures. Suggested controls to mitigate risk might include increasing or decreasing the microbiological or chemical, and changing the controls set according to the process and points criticality. The impact of the mitigation plan may impact several departments, new and old. As a result, the change control plan definition is necessary to structure the actions to be taken by priority and time required. For new cleanrooms, this intervention takes place in an early phase to avoid a snowball of unfixable issues down the line.
Remember that the essential ingredient for contamination control is building a team of experts encompassing quality, production, engineering, and QRM. Use the resources available at your site to build a solid base of process knowledge and take advantage of the QRM experts at Particle Measuring Systems to assist you with the design of your contamination control strategy.
Learn more… Get the full paper.

