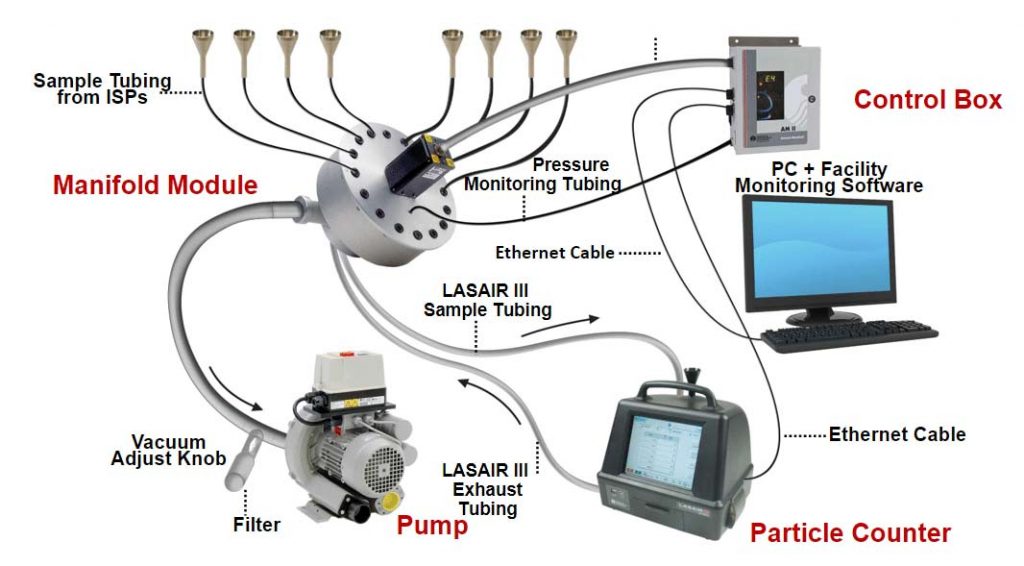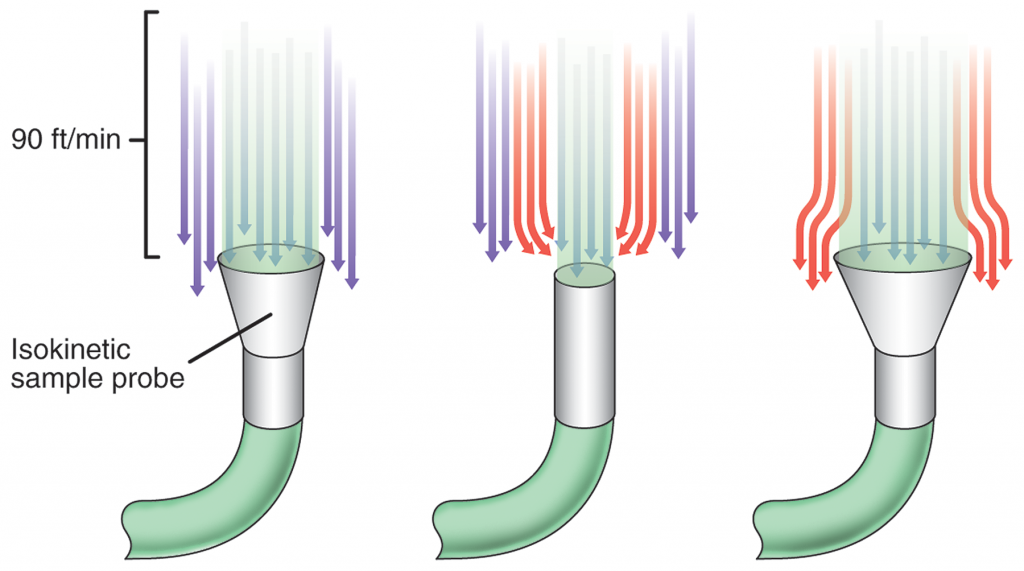
Isokinetic Sampling
In the highly regulated and risk-sensitive world of pharmaceutical manufacturing, adherence to stringent compliance measures is paramount. To achieve this, pharmaceutical companies use controlled environments, particularly cleanrooms, where the risk of variability and particle contamination is minimized. Here we’ll delve into the significance of cleanrooms and explore a key contamination management technique employed within them—Isokinetic Sampling and unidirectional airflow.