Particle Counting in Injectable Solutions 2007 Updates
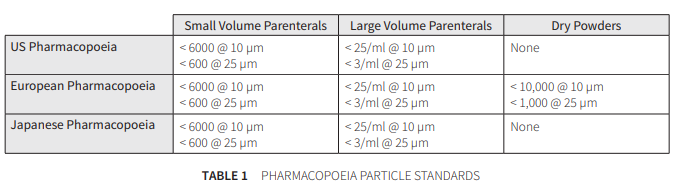 This paper discusses the requirements laid out by US (USP), European (EP), and Japanese (JP) Pharmacopoeia standards and includes the most recent USP <788> (April 2007), EP 5.1 and JP 15 release information. These standards demand that injectable solutions are effectively monitored for micro-contamination, specifically non-soluble particulates.
This paper discusses the requirements laid out by US (USP), European (EP), and Japanese (JP) Pharmacopoeia standards and includes the most recent USP <788> (April 2007), EP 5.1 and JP 15 release information. These standards demand that injectable solutions are effectively monitored for micro-contamination, specifically non-soluble particulates.
Notable changes have occurred to all of the three standards, namely:
- USP recently updated in April 2007 and undertakes a new course on testing.
- EP changed to include SVI products in 2005.
- JP updated to JP in 2006.
Pharmaceutical companies are manufacturers of both solid and liquid formulations. Solid formulations are tablets, dry powers, confectionery, and some solid injectables. Liquid formulations, historically known as parenteral solutions, are now described as injectable solutions or injectables. They include opthalmics, ointments, I.V., vaccines, and others. Injectable solutions are packed as Large Volume Injectable (LVI) solutions, Small Volume Injectable (SVI) solutions, and dry powders requiring reconstitution as either LVI or SVI but most commonly as SVI.
LVIs are typically packaged as bags or bottles containing large volumes of intravenous (IV) solutions. Common uses of LVI solutions without additives include: 1) correction of electrolyte and fluid balance disturbances; 2) nutrition; 3) a vehicle for administering other drugs. Large volume parenteral solutions are packaged in containers holding 100 ml or more (≥ 100 ml) and can be packaged in one of the three types of containers: glass bottle with an air vent tube, glass bottle without an air vent tube, or plastic bags.
Small volume parenteral (SVI) solutions are usually less than 100 ml (< 100 ml) and are packaged depending on the intended use. SVIs are typically packed as ampoules, vials, small bags, and pre-filled syringes.If the solution is a sterile formulation it must be free of all visible particulate material as well as of smaller particles. Particulate material refers to mobile solids unintentionally present in parenteral products. These solids may consist of individual components or mixtures of cellulose, glass, or rubber cores from vials, metal, or plastic fragments. Sterile suspensions may have particulate material but these are usually the active drug or an ingredient, not contaminants
Complete the form for the full paper.

