How a particle travels along tubing between a sample inlet and the particle counter is made messy by a host of physical forces. It’s so messy, validating this sample tubing can be difficult. So what are these forces, what are the losses, and what are the acceptable results?
The Forces Acting on Particles
Cleanroom certification and monitoring activities can be seen as tests performed to quantify the dynamics of the body of air within a confined space. This space may be either the air in the general cleanroom or in a transport duct or a laminar flow zone. The following describes the mechanisms of how particles behave and will assist in the understanding of sampling difficulties and in improving the efficiency of sampling.
The Stokes number is the ratio of a particle’s radius to the dimension of an obstacle to fluid flow. This is an important factor in determining when a particle in motion will be collected by an obstacle or will pass around it. An obstacle could be a filter fiber or the sample inlet.
The drag coefficient is the ratio of the force of gravity to the inertial force on a particle in fluid. It indicates how a particle will resist any force that could cause a change in the particle velocity.
Smaller particles have smaller drag coefficients owing to their lesser mass.
The relaxation time is the time for a particle initially in equilibrium with a moving fluid to match a change in fluid velocity. Large particles have a long relaxation time. Therefore, when an air stream moves through tubing that contains small-radius bends or elbows, the large particles will deposit on a tube wall because they cannot adapt easily to sudden velocity changes owing to tube curvature, but will continue in their original direction until they impact on the tube wall. A related term is stopping distance, which is defined as the distance for a particle initially moving within a gas stream to come to a stop when the gas flow is halted, as by an obstacle.
The deposition velocity or sedimentation velocity is the ratio of particle flux, distance per unit time for sedimentation to occur relative to the ambient particle concentration.
Settling velocities of particles |
|
Particle Size (μm) |
Settling Velocity (cms-1) |
| 0.00037 | – |
| 0.01 | 6.95 x 10-6 |
| 0.1 | 8.65 x 10-5 |
| 1.0 | 3.50 x 10-3 |
| 10 | 3.06 x 10-1 |
| 100 | 2.62 x 10-1 |
There are also additional forces in effect on particles. These forces on the particles and their subsequent response to those forces control the particles migration through the air are:
Viscous forces – The fluid dynamic force from a moving fluid stream. The viscous nature of an air stream will pull particles along that flow path. If this flow is laminar, then other forces act upon the larger particles encouraging settling and deposition. Smaller particles remain buoyant on a laminar flow.
In turbulent flow streams the larger particles are re-entrained back into the airflow should they deposit and smaller particles are now more prone to additional forces acting upon them.
Brownian motion – As the particles migrate through a body of air, random impacts from individual molecules will cause them to veer from course.
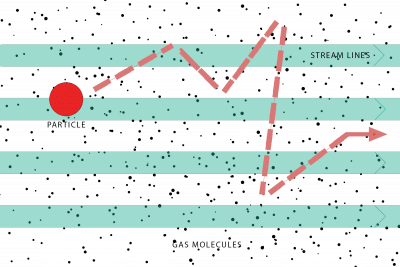
Gravitational force – This force on a particle varies with particle mass and the difference between particle and air density; the larger the particle the greater the interaction.
Electrostatic forces – This force on a particle varies with the particle’s electrical charge (surface area controlled) and the strength of the electrical field in which the particle is located. Electrostatic charge can develop as a particle slips through the air stream. It is important to minimize these interactions to ensure all particles reach the final destination.
Diffusion forces – This force on a particle varies inversely with particle’s radius. Therefore, smaller particles are more prone to interactions due to diffusion.
Thermophoretic forces – These forces (effective mainly for small particles) vary with the particle’s surface area and temperature gradient.
