Pharmaceutical manufacturing environments have traditionally seen particle counting as a burden required to demonstrate compliance to a cleanroom standard. Now, however, Process Analytical Technologies (PAT) changes the focus to be on the final quality of the product. To achieve this, continuous facility monitoring is recommended. This follows the original intent of PAT, which is “to understand and control the manufacturing process: quality cannot be tested into products; it should be built-in or should be by design using a system for designing, analyzing, and controlling manufacturing through process measurements of critical quality and performance attributes of materials and processes with the goal of ensuring final product quality.”
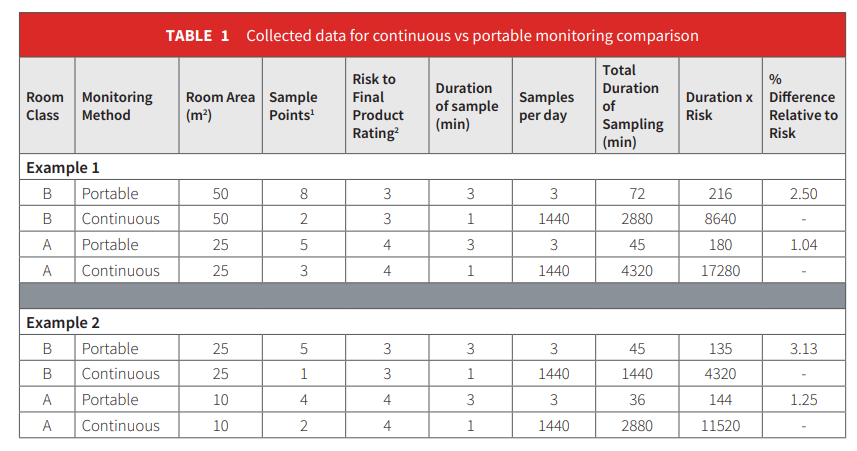
Portable particle counters are very useful in demonstrating that a cleanroom is generally compliant to regulatory limits and certifying to international standards. Occasional particle counting does not, however, catch all instances of contamination in a cleanroom. Contamination trending analysis is imperative to ensure there is minimal risk to product quality. Given the cleanliness techniques used within a pharmaceutical processing environment, the particle sizes of concern, 0.5 and 5.0 micron, are unlikely to enter a cleanroom via the filtration system. Contamination is therefore either a process or personnel issue, both of which can be very fleeting. For example, if a vial is broken and immediately cleaned up, and the incident lasts for less than one minute, it is very unlikely to be noticed by a portable particle counter technique. However, the event will be witnessed using a continuous monitoring system.
Learn more: complete the form to download the full paper…






