The FDA requires HCT/P Environmental Monitoring per the current the current Good Tissue Practice (GTP), which governs the methods used in, and the facilities and controls used for, the manufacture of human tissue and cellular and tissue-based products. The following regulation is to be enforced as of May 25, 2005: 21 CFR 1271—Human Cells, Tissues, and Cellular and Tissue-Based Products 21 CFR 1271.195 Environmental control and monitoring Section 1271 requires facilities to establish and maintain procedures to adequately control and monitor environmental conditions and to provide proper conditions for operations. The regulations were created to improve protection of the public health. Section 1271.195 specifies the monitoring parameters required.
The primary purpose behind these requirements is to prevent the contamination of tissues or tissue/cellular products. The regulations specify that the ventilation and air filtration system require monitoring. Standard monitoring procedures include particle counting and air velocity monitoring within the clean zone and differential pressure monitoring between adjacent areas. Monitoring proves control over products directly exposed to the air quality of the clean zone, and it also protects adjacent rooms from cross-contamination.
 In addition to particle counting, Section 1271.195c also mandates that monitoring for microorganisms should be done when appropriate. The FDA clarifies their expectations in the Federal Register, Vol. 69, Part IV (page 68629):
In addition to particle counting, Section 1271.195c also mandates that monitoring for microorganisms should be done when appropriate. The FDA clarifies their expectations in the Federal Register, Vol. 69, Part IV (page 68629):
Conditions may differ from facility to facility (and even from room to room within a facility), with uncommon microorganisms found in one area but not another. Each establishment should determine the microorganisms that may exist in its facilities and design its monitoring program accordingly. FDA has issued a draft guidance document entitled ‘‘Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing, Current Good Manufacturing Practice,’’ dated August 2003, that may provide useful information to an HCT/P establishment that is developing procedures on environmental control and monitoring. Information on environmental monitoring may also be found in the U.S. Pharmacopoeia.
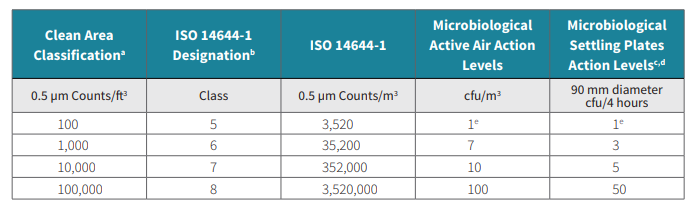
The guidance for Industry was since revised and made into a September 2004 release. That release states that in critical areas, a room particulate classification of < 3,520 particles/m3 at 0.5 µm (ISO14644-1 Class 5) must be maintained, and the supporting clean areas must be ISO 14644-1 class 6, 7, or 8, based upon a risk analysis of operations. The following table outlines ISO classifications for particles and recommended action levels for microbiological quality.
Learn more. Complete the form to download the full paper…





