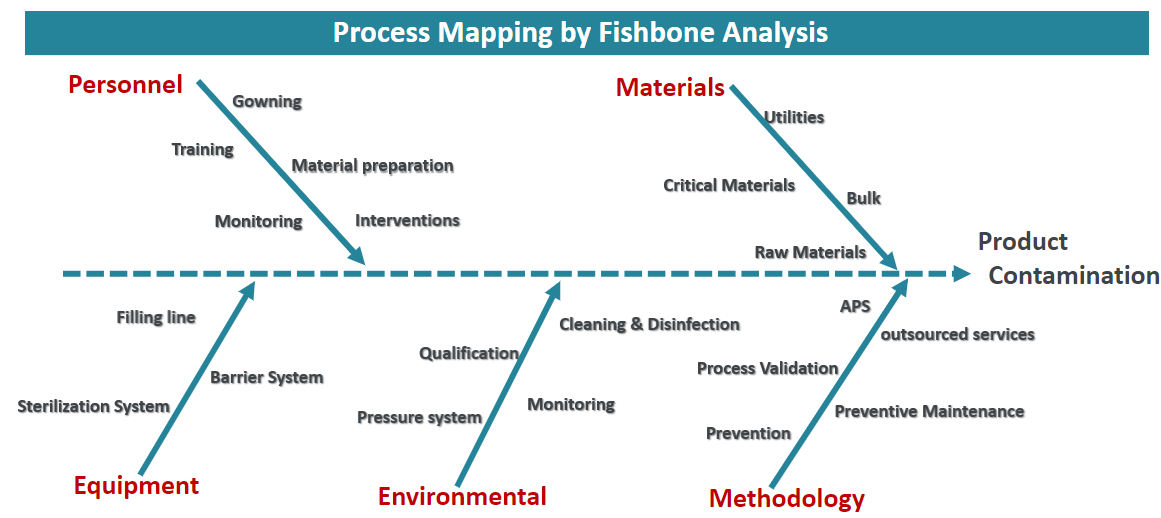
Answers to questions asked during the webinar “Considerations and Steps for Building a Contamination Control Strategy”. Many questions were asked about contamination monitoring requirements in cleanrooms and sampling placement pertaining to process design.
Some contamination control strategy questions covered in this paper are:
- From your experience, does a contamination control strategy (CCS) risk management using FMEA depend on historical data or experience and expectations?
- What is the air classification of a vial sealing/capping area and what is the right location for a non-viable counter?
- How far can airborne sensors be placed from the critical measuring point and how can we know that our points actually give us a good aseptic representation of the cleanroom?
- Many more…..
Examples of answers to the CCS webinar
What are the recommendations for the L-R Method using particle detection in aseptic processing?
The L-R method could be very useful, but it only allows you to see the sampling point trend. For the new filling line, the fluid dynamical studies help to see all possible impacts for contamination. These impacts are not direct, but carried by the turbulence generated above all inside the insulators.
Should we perform a cleaning validation study for the weighing area (surface of the scales, etc)? Is there any minimum holding time necessary for passing through the airlocks (for humans, equipment, raw materials, etc)?
The microbiological cleaning validation must be performed in the rooms representing a worst-case result of an evaluation matrix. Chemical cleaning validation must be done on all equipment in direct contact with the product. Holding time is required and this must be defined with particle recovery studies during the qualification of the environments.
Learn more… Complete the form to download the full paper.