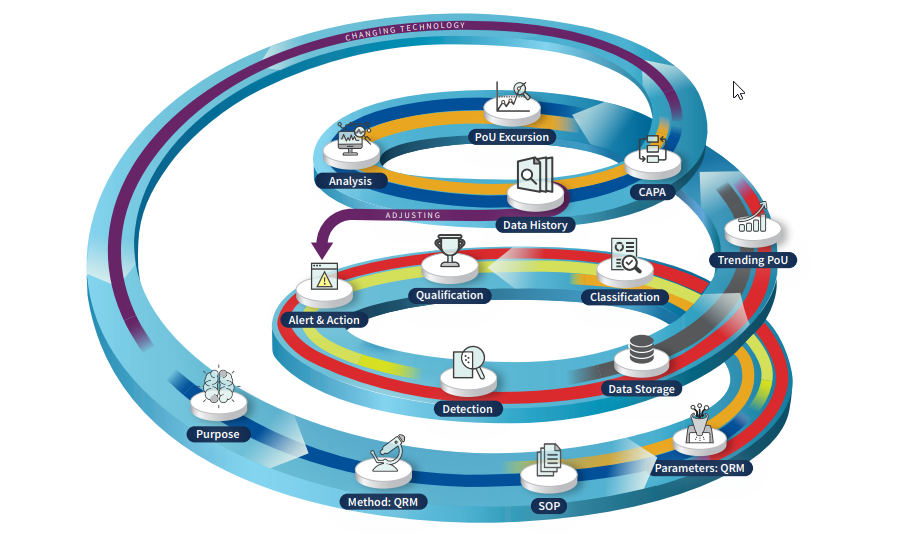
Environmental monitoring data quality is a concept that moves beyond contamination monitoring and builds into effective contamination control.
The role of quality systems in pharmaceutical organizations has grown faster than any other function during the last 15 years. During this period, a small group dedicated to traditional Compliance grew and expanded to include Quality Experts in areas such as validation, product release, operations, sterility assurance, and other specialized functions. Creating a deeper quality connection between manufacturing and engineering has always been the aim of that growth.
Little by little, quality became fundamental to every step of the pharmaceutical manufacturing process: changing from a silo concept to a more fluid one. Now, a new challenge is approaching. The fourth industrial revolution (Pharma 4.0™) is the beginning of the “Smart Facility” era, where digitalization and automation will combine to reach very complex applications and life cycles. In this brand-new framework, Quality Experts will face the challenge of rethinking their roles and redesigning the Quality Systems of their pharmaceutical companies to be based on the concepts of Data Quality.
The ICH Idea of Pharma
Since 1990, the International Council for Harmonization (ICH) has aimed to achieve a unique response worldwide to ensure that safe, effective, and high-quality medicines are developed and registered in the most resource-efficient manner. Between 2005 and 2008, with the publication of the Q9 and Q10 guidelines, the council explained what the quality systems should be and how they should work to assure the highest possible quality drug product.
During these years, we can affirm that most of the pharmaceutical firms in the world designed their quality system to fit the ICH guidelines, enhancing the ICH’s role in the pharmaceutical organization. Simultaneously the value and the challenge of the ICH was, fundamentally, to use science and risk-based quality system principles to cover the entire manufacturing product life cycle.
Learn more… Complete the form to download the full paper.