USP and its Implications for Measuring Microbial Recovery Rates
The recently revised United States Pharmacopoeia (US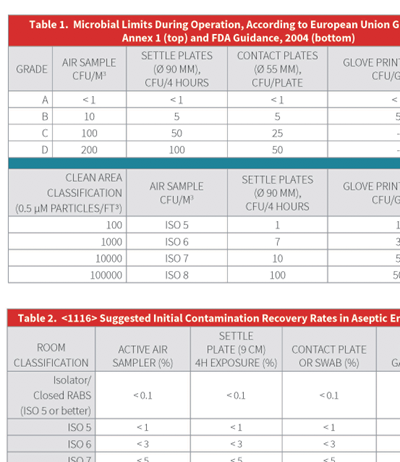 P) chapter <1116> Microbial Control and Monitoring of Aseptic Processing Environments Chapter <1116> is arguably one of the most comprehensive informational chapters from the USP, and it is particularly challenging due to its proposal regarding measurement of microbial contamination based on Contamination Recovery Rates (CRR) rather than the conventional enumeration of colony forming units (CFU).
P) chapter <1116> Microbial Control and Monitoring of Aseptic Processing Environments Chapter <1116> is arguably one of the most comprehensive informational chapters from the USP, and it is particularly challenging due to its proposal regarding measurement of microbial contamination based on Contamination Recovery Rates (CRR) rather than the conventional enumeration of colony forming units (CFU).
This paper covers
- Main changes from the previous revision
- Recommendations when using CRR
- Case Study
- Conclusions on CRR
This paper also includes recommendations for monitoring solutions to meet regulatory requirements including the MiniCapt Mobile for Microbial Monitoring and the BioCapt Single Use Microbial Impactor, which can be used with the MiniCapt Mobile for a fully validated system.
Authors: Gilberto Dalmaso, Ph.D. and Claudio Denoya, Ph.D.
Complete the form to receive your free copy of this paper.

