Counting Microplastics in Wastewater
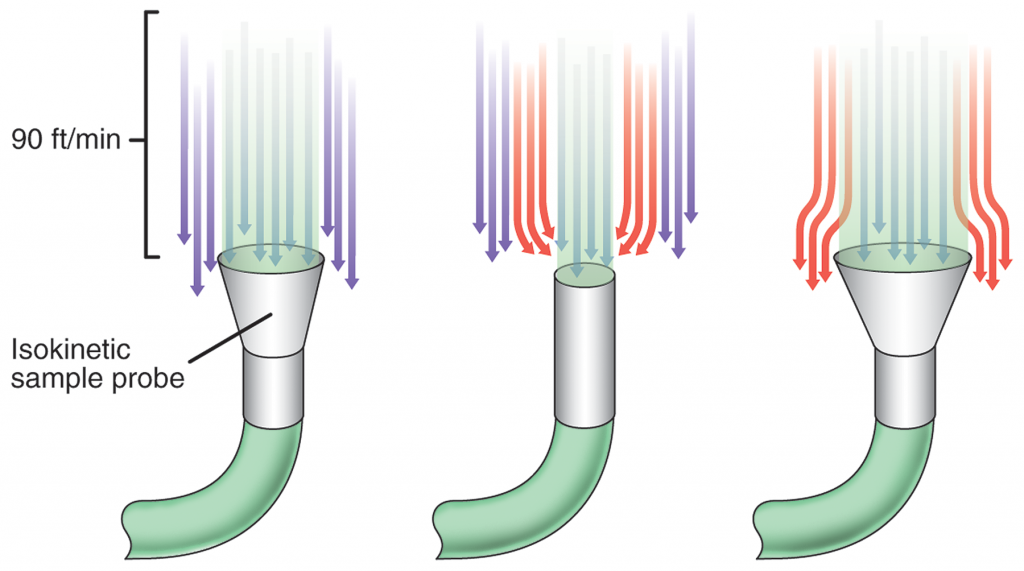
This paper discusses the goal to minimize microplastics in aquatic systems and validating the results. The authors of this paper propose a new approach of measurement and filtration that has the opportunity to reduce the microplastics that end up in our water. This alternative method would allow manufacturers to measure particles in treated textile water continuously for particles that are as small as 2.0 microns.